Synthesis of Physiological Active Compounds Laboratory
|
|
CONTACTS |
|
|
||
|
Olga Yu. Reshmina
|
MAIN AREAS OF RESEARCH AND DEVELOPMENT
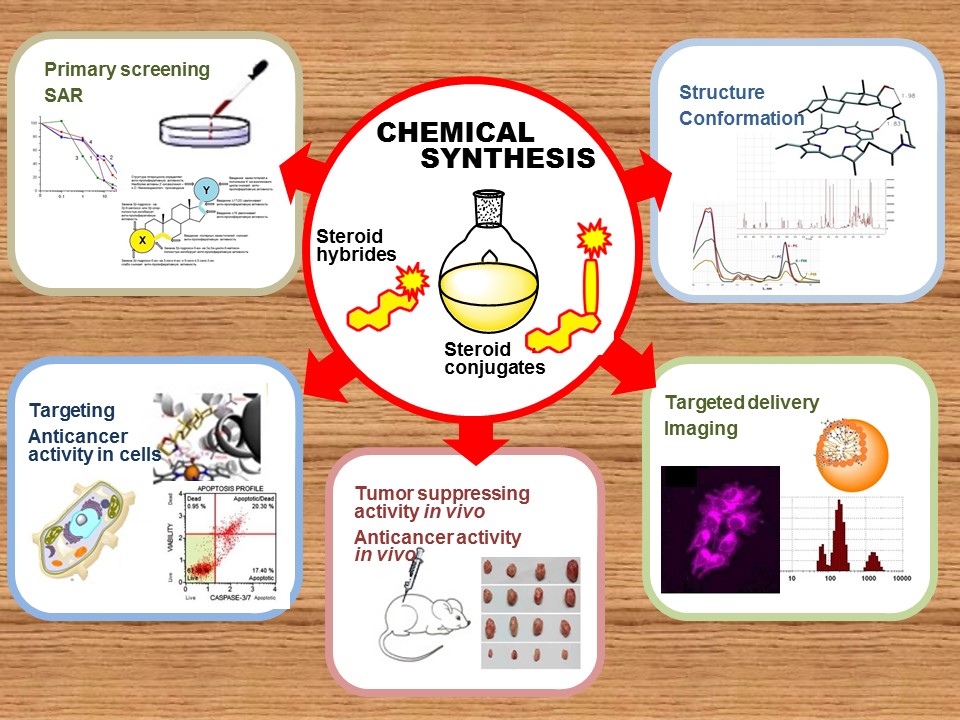
Currently, the main scientific direction of the Laboratory's work is design, chemical synthesis, structural studies, research of biological activity and pharmacological potential of new anticancer agents - steroid hybrids and conjugates.
The Laboratory carries out work on the chemical synthesis of hybrids and conjugates of steroids; studing of the structure and conformation of the resulting compounds; their interaction with cancer-transformed cells; searching for the most important biotargets; assessment of their antiproliferative and antitumor activity in cell culture in vivo.
Synthesis of Phisiological Active Compounds Laboratory is the oldest IBMC science department, founded in 1945 by famed Soviet chemist – academician M. M. Shemyakin. Number of prominent scientists worked in the laboratory over the years: M.N. Kolosov, V.K. Antonov, E.S. Chaman, V.F. Pozdnev, G.V. Ponomarev and others.
Laboratory specialization is the medicinal and organic chemistry, natural compounds and their synthetic analogies.
Current scientific direction of the laboratory is the search and development of anti-cancer agents, based on steroids hybrides and conjugates.
EDUCATIONAL ACTIVITIES
The Laboratory is a platform for student’s scientific works and performing of baccalaureate, magisterial and diploma projects of graduates from Mendeleev University of Chemical Technology, Moscow Technological University, Pirogov Medical University.
Researchers of the Laboaratory conduct lecture courses on various branches of chemistry in leading Russian universities.
MAIN ACHIEVEMENTS
- Priority research in the area of antibiotics and amino acids (1945–1961)
- Discovery of vitamin B6 and development of the foundations of pyridoxal catalysis (1951–1961)
- Creation of original (the second in the world) peptide synthesizer and solid-phase synthesis of bradykinin (1966 – 1970)
- Priority research in the area of pyrocarbonate chemistry, peptide synthesis and peptidomimetics (1969–2019)
- Priority research in the area of tetrapyrrole heterocycles and their metal complexes (1990–2020)
- Priority research in the area of synthetic oxysterols and their analogues (2006–2010)
- Priority research in the area of nitrogen-containing pregnane steroids, development of the antitumor agent alseviron and its preclinical studies (2013 – 2020)
- Priority research in the area of steroid hybrids and conjugates, development of the antitumor agent misolacron (2019 – 2023)
PROJECTS
- RFBR grant 20-515-00021 Bel_а “Design, synthesis and biological investigation of 21-norsteroid 20-аzoles as new antagonists of androgen and estrogen receptors in MCF-7 and LNCaP cells”
- RSF grant 18-75-10008 (in collaboration with Blokhin Cancer Research Center)
- RFBR grant 20-515-00021 Bel_а “Design, synthesis and biological investigation of 21-norsteroid 20-аzoles as new antagonists of androgen and estrogen receptors in MCF-7 and LNCaP cells”
- RFBR grant 15-04-03970 “The role of membrane lipids in the aggregation of beta-amyloid”(in collaboration with IMB RAN)
- RFBR grant 15-04-02939 “Novel nitrogen-containing derivatives of 17(20)E-pregnene as androgens metabolism regulators in prostate carcinoma cells”
- RFBR grant 15-04-02426 “Design and synthesis of bifunctional conjugates of pyropheophorbide a and their application for PDT of pathogens and tumors”
- State contract № 14.N08.11.0107 “Preclinical investigation of pharmaceutical substance based on oxazolinyl derivative for prostate cancer therapy”
![]() Korolchuk A.M., Zolottsev V.A., Misharin A.Y. (2023) Conjugates of Tetrapyrrolic Macrocycles as Potential Anticancer Target‑Oriented Photosensitizers, Topics in Current Chemistry, 381(2), 10. DOI:10.1007/s41061-023-00421-0
Korolchuk A.M., Zolottsev V.A., Misharin A.Y. (2023) Conjugates of Tetrapyrrolic Macrocycles as Potential Anticancer Target‑Oriented Photosensitizers, Topics in Current Chemistry, 381(2), 10. DOI:10.1007/s41061-023-00421-0
![]() Latysheva A.S., Zolottsev V.A., Veselovsky A.V., Scherbakov K.A., Morozevich G.E., Zhdanov D.D., Novikov R.A., Misharin A.Y. (2023) Oxazolinyl derivatives of androst-16-ene as inhibitors of CYP17A1 activity and prostate carcinoma cells proliferation: Effects of substituents in oxazolinyl moiety, Journal of Steroid Biochemistry and Molecular Biology, 230, 106280. DOI:10.1016/j.jsbmb.2023.106280
Latysheva A.S., Zolottsev V.A., Veselovsky A.V., Scherbakov K.A., Morozevich G.E., Zhdanov D.D., Novikov R.A., Misharin A.Y. (2023) Oxazolinyl derivatives of androst-16-ene as inhibitors of CYP17A1 activity and prostate carcinoma cells proliferation: Effects of substituents in oxazolinyl moiety, Journal of Steroid Biochemistry and Molecular Biology, 230, 106280. DOI:10.1016/j.jsbmb.2023.106280
![]() Khan I.I., Karshieva S.S., Sokolova D.V., Spirina T.S., Zolottsev V.A., Latysheva A.S., Anisimova N.Y., Komarova M.V., Yakunina M.N., Nitetskaya T.A., Misharin A.Y., Pokrovsky V.S. (2022) Antiproliferative, proapoptotic, and tumor-suppressing effects of the novel anticancer agent alsevirone in prostate cancer cells and xenografts, Archiv der Pharmazie, 355(1), 2100316. DOI:10.1002/ardp.202100316
Khan I.I., Karshieva S.S., Sokolova D.V., Spirina T.S., Zolottsev V.A., Latysheva A.S., Anisimova N.Y., Komarova M.V., Yakunina M.N., Nitetskaya T.A., Misharin A.Y., Pokrovsky V.S. (2022) Antiproliferative, proapoptotic, and tumor-suppressing effects of the novel anticancer agent alsevirone in prostate cancer cells and xenografts, Archiv der Pharmazie, 355(1), 2100316. DOI:10.1002/ardp.202100316
![]() Zolottsev V.A., Latysheva A.S., Khan I.I., Pokrovsky V.S., Misharin A.Y. (2022) Design and Synthesis of New Agents for Prostate Cancer Treatment Inspired by Steroidal CYP17 A1 Inhibitors, ChemistrySelect, 7(45), e202203393. DOI:10.1002/slct.202203393
Zolottsev V.A., Latysheva A.S., Khan I.I., Pokrovsky V.S., Misharin A.Y. (2022) Design and Synthesis of New Agents for Prostate Cancer Treatment Inspired by Steroidal CYP17 A1 Inhibitors, ChemistrySelect, 7(45), e202203393. DOI:10.1002/slct.202203393
![]() Zolottsev V.А., Latysheva А.S., Pokrovsky V.S., Khan I.I., Misharin A.Y. (2021) Promising applications of steroid сonjugates for cancer research and treatment, European Journal of Medicinal Chemistry, 210, 113089. DOI:10.1016/j.ejmech.2020.113089
Zolottsev V.А., Latysheva А.S., Pokrovsky V.S., Khan I.I., Misharin A.Y. (2021) Promising applications of steroid сonjugates for cancer research and treatment, European Journal of Medicinal Chemistry, 210, 113089. DOI:10.1016/j.ejmech.2020.113089
![]() Latysheva A.S., Zolottsev V.A., Pokrovsky V.S., Khan I.I., Misharin A.Yu. (2021) Novel nitrogen containing steroid derivatives for prostate cancer treatment, Current Medicinal Chemistry, 28(40), 8416-8432. DOI:10.2174/0929867328666210208113919
Latysheva A.S., Zolottsev V.A., Pokrovsky V.S., Khan I.I., Misharin A.Yu. (2021) Novel nitrogen containing steroid derivatives for prostate cancer treatment, Current Medicinal Chemistry, 28(40), 8416-8432. DOI:10.2174/0929867328666210208113919
![]() Latysheva A.S., Zolottsev V.A., Veselovsky A.V., Scherbakov K.A., Morozevich G.E., Pokrovsky V.S., Novikov R.A., Timofeev V.P., Tkachev Y.V., Misharin A.Y. (2020) New steroidal oxazolines, benzoxazoles and benzimidazoles related to abiraterone and galeterone, Steroids, 153(1), 108534. DOI:10.1016/j.steroids.2019.108534
Latysheva A.S., Zolottsev V.A., Veselovsky A.V., Scherbakov K.A., Morozevich G.E., Pokrovsky V.S., Novikov R.A., Timofeev V.P., Tkachev Y.V., Misharin A.Y. (2020) New steroidal oxazolines, benzoxazoles and benzimidazoles related to abiraterone and galeterone, Steroids, 153(1), 108534. DOI:10.1016/j.steroids.2019.108534
![]() Zolottsev V.A., Ponomarev G.V., Taratynova M.O., Morozevich G.E., Novikov R.A., Timofeev V.P., Solyev P.N., Zavialova M.G., Zazulina O.V., Tkachev Y.V., Misharin A.Y. (2018) Conjugates of 17-substituted testosterone and epitestosterone with pyropheophorbide a differing in the length of linkers, Steroids, 138, 82-90. DOI:10.1016/j.steroids.2018.06.011
Zolottsev V.A., Ponomarev G.V., Taratynova M.O., Morozevich G.E., Novikov R.A., Timofeev V.P., Solyev P.N., Zavialova M.G., Zazulina O.V., Tkachev Y.V., Misharin A.Y. (2018) Conjugates of 17-substituted testosterone and epitestosterone with pyropheophorbide a differing in the length of linkers, Steroids, 138, 82-90. DOI:10.1016/j.steroids.2018.06.011
![]() Taratynova M.O., Zolottsev V.A., Tkachev Ya.V., Novikov R.A., Zavialova M.G., Morozevich G.E., Timofeev V.P., Romanenko Yu.V., Koifman O.I., Misharin A.Y., Ponomarev G.V. (2018) Trifunctional (Pyropheophorbide a – Steroid – Hexadecyl Chain) Conjugates: Synthesis, Solubilization, Interaction with Cultured Cells, Macroheterocycles, 11(3), 277-285. DOI:10.6060/mhc180999p
Taratynova M.O., Zolottsev V.A., Tkachev Ya.V., Novikov R.A., Zavialova M.G., Morozevich G.E., Timofeev V.P., Romanenko Yu.V., Koifman O.I., Misharin A.Y., Ponomarev G.V. (2018) Trifunctional (Pyropheophorbide a – Steroid – Hexadecyl Chain) Conjugates: Synthesis, Solubilization, Interaction with Cultured Cells, Macroheterocycles, 11(3), 277-285. DOI:10.6060/mhc180999p
![]() Kostin V.A., Zolottsev V.A., Kuzikov A.V., Masamrekh R.A., Shumyantseva V.V., Veselovsky A.V., Stulov S.V., Novikov R.A., Timofeev V.P., Misharin A.Y. (2016) Oxazolinyl derivatives of [17(20)E]-21-norpregnene differing in the structure of A and B rings. Facile synthesis and inhibition of CYP17A1 catalytic activity, Steroids, 115, 114-122. DOI:10.1016/j.steroids.2016.06.002
Kostin V.A., Zolottsev V.A., Kuzikov A.V., Masamrekh R.A., Shumyantseva V.V., Veselovsky A.V., Stulov S.V., Novikov R.A., Timofeev V.P., Misharin A.Y. (2016) Oxazolinyl derivatives of [17(20)E]-21-norpregnene differing in the structure of A and B rings. Facile synthesis and inhibition of CYP17A1 catalytic activity, Steroids, 115, 114-122. DOI:10.1016/j.steroids.2016.06.002
Laboratory actively and fruitfully cooperates with Russian and foreign scientific institutions. Among the scientific collaborators of Laboratory are top researchers from:
- Engelgardt Institute of Molecular Biology RAN, Moscow
- Biological Faculty of Moscow State University, Moscow
- National Medical Research Center "Oncology named after N.N. Blokhin", Moscow
- Institute of Bioorganic chemistry NANB, Minsk
- Medicine Faculty of Patrice Lumumba Peoples’ Friendship University of Russia, Moscow
- Pregn-17(20)-еne derivatives exhibited anti-tumor activity. Patent RU2015111223A.
- Pharmaceutical composition for luminescent diagnostics of pathologic alterations of skin and mucosa. Patent RU2016107576A
- Method of obtaining diethrye salt 2,4-di(1-metoxyethyl)deuteroporphyrin-IX (Dimegin). Patent RU2647588 C1.
- Photosensitizer for therapy of oncologic diseases and the method of its preparation. Patent RU2646477C1.
- Method of preparation of dikalium salt ytterbium complex of 2,4-di(1-metoxyethyl)deuteroporphyrin-IX acetyl acetonate Patent RU2697418 С1.
The laboratory is a platform for student’s scientific works and performing of baccalaureate, magisterial and diploma projects of graduates from Mendeleev University of Chemical Technology, Moscow Technological University, Pirogov Medical University
LIST OF STUDENT’S PROJECTS PERFORMED IN LABORATORYLIST OF STUDENT’S PROJECTS PERFORMED IN LABORATORY(2016–2020)
- Zazulina О.V. «Synthesis of main steroid blocks for the preparation of antiandrogens» (baccalaureate work, Mendeleev University of Chemical Technology, 2016.)
- Yanova Т.I. “Biological activity of biosynthetic precursors of brassinosteroids and related derivatives as potential anti-tumor agents (diploma work, Pirogov Medical University, 2017.)
- Zazulina О.V. «Synthesis of nitrogen containing derivatives of 17β-hydroxypregnanic and 17α-hydroxypregnanic acids – novel potential antiandrogens» (magisterial dissertation, Mendeleev University of Chemical Technology 2018)
- Taratynova М. О. «Novel polyfunctional conjugates of testosterone with pyropheophorbide a – synthesis and biological activity» (baccalaureate work, Moscow Technological University, 2018)
- Latysheva А.S. «Inhibition of 17α-hydroxylase/17,20-lyase by nitrogen containing derivatives of [17(20)E]-pregnene» (diploma work, Pirogov Medical University, 2018)
- Scherbakov S.А. «Synthesis of nitryl and oxazolinyl derivatives of Δ16-pregnene – new potential antiandrogens» (baccalaureate work, Mendeleev University of Chemical Technology, 2018)
- Меstahudinova J.А. «Synthesis of steroid blocks for preparation of new inhibitors of Hh- signaling» (baccalaureate work, Mendeleev University of Chemical Technology, 2020)
- Маtiusova Е.V. «Synthesis of seco-D-androstane and seco-D-21-norpregane derivatives – precursors for the preparation of new antiandrogens» (baccalaureate work, Mendeleev University of Chemical Technology, 2020)
- Kutdusova G.R. “Synthesis of 6β-methoxy-16-amino-17-hydroxy-3α,5α-cyclo-16,17-secoandrostane - a precursor for the production of potential androgen receptor destructors (course project, M.V. Lomonosov Moscow State University, 2021)
- Korolchuk A.M. “Functionally oriented synthesis of amide derivatives of 21-norpregn-17(20)-ene and Δ5,16-androstane - potential androgen receptor antagonists” (diploma, Mendeleev University of Chemical Technology, 2021)
- Sdobnikova A.A. “Design and synthesis of new antitumor agents based on nitrogen-containing derivatives of 16,17-secosteroids” (magisterial dissertation, Mendeleev University of Chemical Technology, 2021)
- Mestakhudinova Yu.A. “Synthesis and biological activity of new generation antiandrogens based on 15-substituted derivatives of seco-D-androstane” (master’s thesis, D. I. Mendeleev Russian Chemical Technology University, 2022)
- Matusova E.V. “Synthesis and biological activity of nitrogen-containing conjugates of seco-D-androstane - potential destructors of the androgen receptor” (magisterial dissertation, Mendeleev University of Chemical Technology, 2022)
- Shishulin D.A. “Synthesis and study of the biological activity of new photosensitizers based on seco-steroids with tetrapyrrole macrocycles” (baccalaureate project, Mendeleev University of Chemical Technology, 2023)
PH.D. THESES PREPARED IN THE LABORATORY
- Zolottsev V. A. ” Synthesis of nitrogen-containing derivatives of the pregnane series - potential antitumor agents ” (02.00.03 –Organic chemistry, 2019 г.)